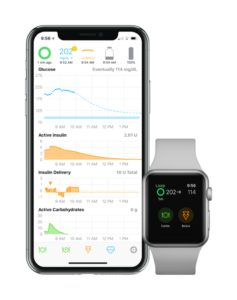
On January 24 the FDA approved Tidepool’s Loop algorithm that automates insulin dosing (*). The FDA approval process took two years and represents a first … a regulated and commercially available solution for patient choice in the algorithms and hardware they instruct. Until this announcement, hardware providers developed and controlled the software in an integrated technology solution.
FDA Approved Loop
Loop works with patient-chosen CGM and insulin pump technology. Other automated insulin solutions are embedded in insulin pump hardware with proprietary software algorithms that don’t permit patient control of certain assumptions. For example, Tandem Diabetes Control IQ has a hard-coded 5-hour insulin duration and a 60% bolus correction.
“We Are Not Waiting” Loop

Why Loop?
But there was so much I liked about the Loop algorithm. I chose the targeted blood sugar levels and insulin duration. It worked with my proven basal profile and made modifications to maintain blood glucose levels in a tight range. Post prandial (after meal) blood sugars were similar to my open loop numbers (this is where Control IQ and Medtronic’s Auto Mode failed me). Loop did not work well when exercising, so I turned it off while working out. My A1C and time in range remained excellent. When the technology worked, it reduced the stress and mindshare of managing glucose levels. In those moments, I trusted it.
Diabetes tech is moving fast these days … such a welcome change from previous decades. In this post Closed Looping – Round III I discuss interest in Medtronic’s 780g due to expanded patient input (it’s not yet available in the U.S.). Now I’m also looking forward to the FDA-approved Loop solution once it announces its’ technology partners. I am particularly interested in the modifications the FDA required to make it commercially available.
I appreciate the creativity and commitment of Howard Look, the Founder and CEO of Tidepool. He’s embraced a Who’s Who of board members, advisors, and outside funding sources that have paved the way for patient choice in software and hardware technology. I’m also grateful for our type 1 community that has gotten behind the ‘We’re Not Waiting’ movement.
For more on Tidepool, check out this link https://www.tidepool.org/
(*) The FDA approval is for type 1s age six and older. Initially, it will work with Apple’s iOS software on iPhones. Versions to work on the Android platform will be available later.